UDI Labeling Compliance System Released
Special support and pricing for UDI Early Adopters
COLUMBUS, Ohio, Oct. 10, 2017 /PRNewswire/ — Automatic Identification Systems, Inc. (AIS) is proud to announce availability of SCANALYST 3 UDI, a Compliance system designed to help the US healthcare industry implement FDA UDI and DSCSA regulations.
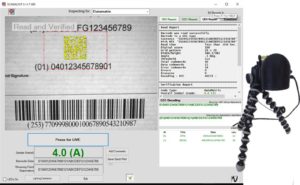
Showing the user interface with a color coded report for a datamatrix barcode. Application Identifiers and field data broken out and checked for compliance. (PRNewsfoto/Automatic Identification System)
Healthcare manufacturers and providers are working toward improving their data infrastructure. The change includes use of standardized product identification in barcodes as well as the use of fast, accurate, proven scanning technology for data collection. This change is required to build a solid data foundation for improving patient safety, reducing hospital’s costs and building a solid Big Data system for further improvements.

The Right Tool for UDI Compliance – UDI compliance requires the barcode to meet minimum quality standards as well as size and format specifications. Finally, the UDI must be on file with the FDA in their GUDID reference catalog. SCANALYST 3 UDI inspects for and records all that. (PRNewsfoto/Automatic Identification System)
Compliance with labeling rules means meeting print quality requirements for barcodes, data content and format as specified and finally, a correct entry in the FDA Global UDI Database that matches the physical product and it markings. The SCANALYST 3 UDI system makes these compliance checks straightforward for manufacturers, distributors and hospitals. The system provides a database for inspection results as well as an integrated email tool for collaboration with trading partners and regulators.
UDI marked product are flowing into the providers at this time and early adopters are starting to take advantage of the availability. To help early adopters, AIS has established a support program as well as special pricing for their systems. “We are committed to help the healthcare industry move through this historical digital transformation. With our systems and our expertise in the AIDC technologies, we can simplify their responsibilities,” said Jeff Nolan, principle at AIS.
About the FDA UDI and DSCSA Regulations:
UDI (Unique Device Identification) and DSCSA (Drug Supply Chain Security Act) cover medical devices and pharmaceutical products respectively. Enacted in 2013 and being enabled now and over the next several years, these programs will significantly improve the healthcare industries ability to deal with today’s challenges. GUDID is the FDA database for all medical devices.
About Automatic Identification Systems, Inc:
AIS is an Ohio company founded in 1985 to assist clients in using the AIDC technologies such as barcode, RFID and IOT. AIS is the developer of the various SCANALYST systems that have been in use around the world since 1993. www.scanalyst.com/udi-compliance
Contact:
Jeff Nolan – AIS
614-431-3300
mktg@ais-co.com
Automatic Identification Systems, Inc.